Style Sampler
Layout Style
Search News Posts
General Inquiries 1-888-555-5555
•
Support 1-888-555-5555
Modeling Disease Spread
Computer-based disease spread models are frequently used in veterinary science to simulate disease spread. They are used to predict the impacts of the disease, plan and assess surveillance, or control strategies, and provide insights about disease causation by comparing model outputs with real life data. There are many types of disease spread models, and here we present and describe the implementation of a particular type: individual-based models.
Disease spread models are simplified representations of real-life systems. Model outputs can only be as accurate as model inputs allow.
The COVID-19 pandemic has brought new attention to models of infectious disease. These models are critical tools that scientists use to anticipate health care needs and explore options for responding to an outbreak. – As illustrated by coronavirus disease 2019 (COVID-19), epidemic models are powerful health policy tools critical for disease prevention and control.
A disease spread model is a simplified representation of a real-life system of disease transmission.
Spread models (also known as mechanistic models of disease spread) include explicit hypotheses of the biological mechanisms that drive infection dynamics. Therefore, they differ from statistical models such as regression models. Disease spread models are motivated by a need to better understand the transmission dynamics of a disease, predict the spread of the disease in a population and its effects, and study how the spread can be influenced (including the evaluation of different strategies to improve surveillance and control of diseases).
. For example, experiments on disease transmission and control might insufficiently represent real-life disease ecology, or not be feasible due to high resource requirements (such as time and monetary costs), or logistical and ethical constraints. In addition, observational studies of disease spread might not provide comparisons of the relevant control strategies, or not occur in the population of interest (e.g., transboundary diseases).
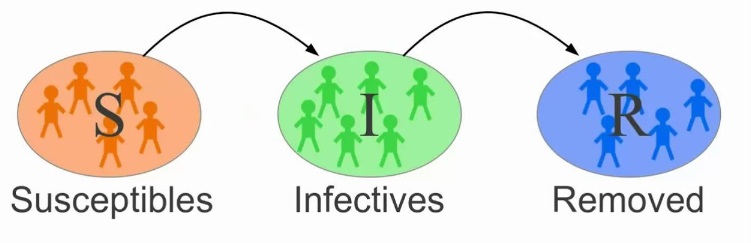
Disease spread models represent the dynamics of infection, or progression of the modeled units of interest through disease states, for instance Susceptible (S), Infectious (I), and Recovered (R) states (an SIR model).
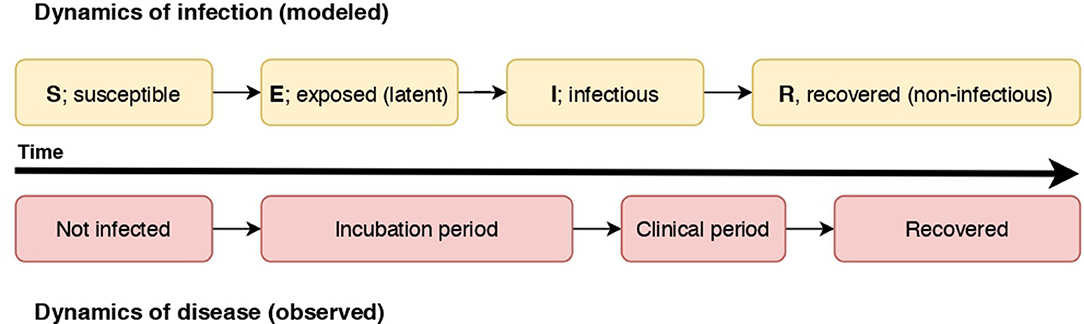
Below diagram illustrating the relationship of the dynamics of an SEIR infectious process and observed disease states in individuals. In this case, individuals become non-infectious prior to resolution of clinical signs.
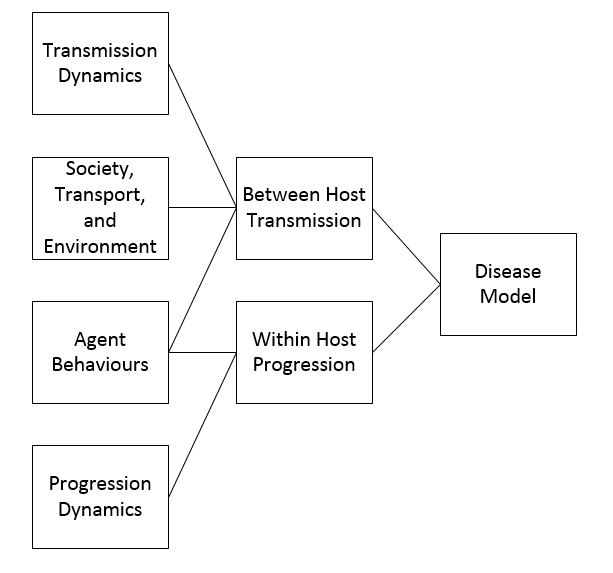
In the flowchart below, Stages, and steps within each stage, in building a disease spread model. AS you can see, diease modeling is a complex task.
The way in which the units of interest contact each other, or how they “mix,” is a core component of a disease model. Homogeneous contact means that all the units have equal probability of contact with each other (no clustering). Heterogeneous contact means that the probability of contact between units of interest is not equal, hence clustering (spatial or related to other contact characteristics) exists in the population. Heterogeneous contact can be modeled by stratifying models into population groups (for example, by age or farm type), modeling contacts between units of interest according to a network structure, or modeling specific characteristics of units that influence contact [for example, furious rabies in dogs;
Initially when constructing a spread disease, the host population dynamics are modeled as the “background” for the disease dynamics. For example, a model of canine rabies spread requires a population of dogs or a foot-and-mouth disease model the population of cloven-hoofed animals. An understanding of the population of interest's demographics are critical. Whilst demographic data for livestock populations can often be gained from government or industry sources, it might be necessary to conduct studies of other populations (such as companion animals) prior to modeling to for example determine age structure and birth and death rates.
The parameters of the society being modelled have a major effect on how a disease will spread between hosts. A densely populated area will result in more contacts between agents, and thus a greater likelihood of infection (Perez & Dragicevic 2009). The social networks of agents also influence the disease spread. In their model of a disease spreading through a small Australian town, Skvortsov et al. (2007) found that the majority of infections in their model occurred at the schools. This was because every agent at a school was in contact with every other agent at the school. The large social networks of agents in the model led to a higher infection rate.
The process of disease transmission is the core dynamic process in the model. Generally, transmission can be considered as either direct (from host to host) or indirect, for example via the environment or vector transmitted (45). It can also be dependent on model features that increase contact heterogeneity; for example, some models are spatially explicit and the probability of transmission varies according to distance, mimicking a system in which transmission varies with spatial location
Our aim is to provide a practical introduction to building individual-based disease spread models.